How to be sure that your Data Integrity project has fully covered your eData LifeCycle?
The integrity of your Data along their lifecycle
This is not another data integrity article, yet this is about demonstrating to auditors that you do have it all right and under control. So we’d like to highlight the importance of assessing the complete life cycle of your data and managing it for complete compliance to data integrity requirements.
We’ve all attended the thousand trainings, webinars and presentation about Data Integrity recommendations and guidelines. We know by heart the ALCOA definition and read several scary 483 letters.
You probably have also been involved in a specific DI project in your company or simply been asked to attend a series of internal training courses to increase self-awareness about the impacts of your actions. Corporate policies and messaging reinforced with nice posters have also probably popped up and decorated nicely the corridors and the cafeteria.
Beside the human factor being approached by reinforcing corporate ethical rules, the integrity of your data is a matter of managing adequately the company data lifecycle and in its multiple data workflows.
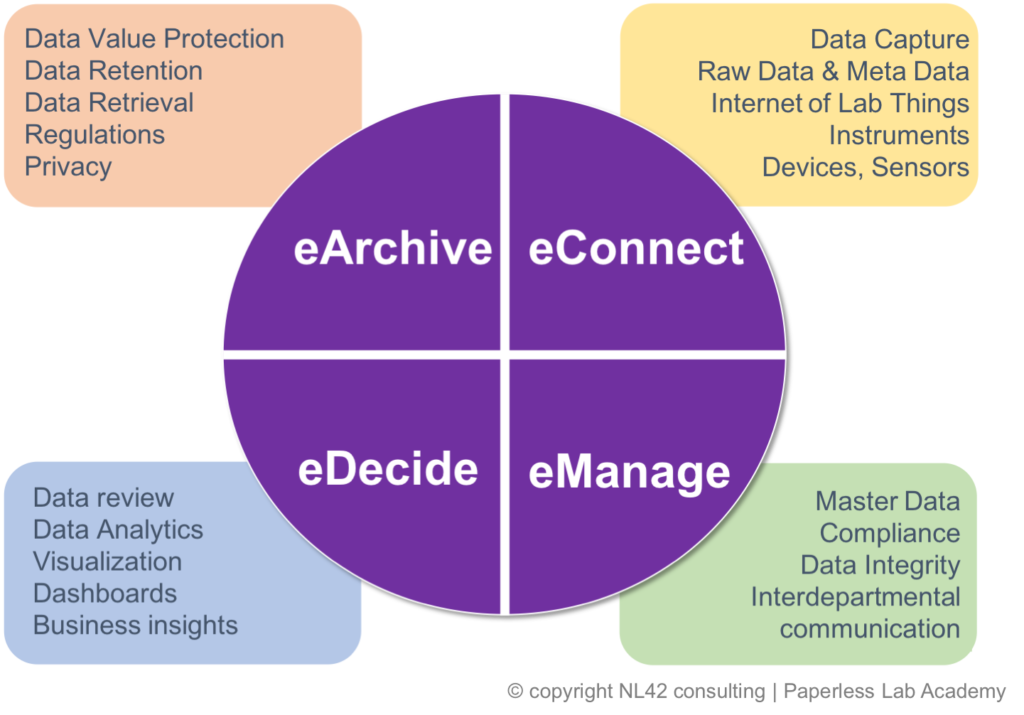
We, at NL42 ,are known at the Paperless Lab Academy for reinforcing the concept that no digital transformation project goes without a clear understanding of your data life cycle which we organise in 4 main steps when speaking about electronic data: eData, we will focus on the initial raw data capturing at eConnect, correct and compliant handling at eManage, easy access to data insight for sound decision making at eDecide .
Two major concerns the auditors will focus on
When investigating possible Data Integrity breaches, auditors are in the end, just using common sense and applying an implacable logic on “HOW” your data are managed. They will tend more and more to prefer seeing digital solutions implemented, with CFR21/11 capabilities enabled, correct users´ right access definitions and more. Even if all possible human interactions prone to transcription errors are removed, there are still two major concerns the auditors will focus on.
1) Accessing the original raw data and its metadata
The eConnect step is becoming of crucial importance as the attention of the auditors is moving to laboratory instrumentation and capabilities to provide electronic format of their data ouput. Raw data and corresponding metadata should be accessible in order to verify easily non only the final result outcome but to review the whole data processing itself too.
Additionally, to the capability to review the data processing, all related information about quality control records, exceptions and re-evaluation should be perfectly accessible anytime.
Curiously for the past 10 years, even though the laboratory information management systems have considerably improved their interfacing capabilities with the laboratory instruments, the instrument integration tend to fall always in a phase 2 implementation and most of the time not being implemented at all.
2) Assessing the Vendor, Documenting and Validating the computerised systems
Assessing the vendor´s product development quality system, documenting from the very first moment of the project planning to ease the validation steps is again a strong recommendation from our side.
Unfortunately, this had to be reinforced again recently by the EMA. In April 2020, the EMA has released a “Notice to sponsors on validation and qualification of computerised systems used in clinical trials” that could easily be extended to any scientific data management workflow still not matured enough regarding data integrity management.
The integrity of your paperless processes is built on the foundation of thorough understanding of your data lifecycle and precise documentation for qualifying the functionalities and validate the reliability and robustness of your computerised system in managing your data. Failure to document and therefore demonstrate the validated state of a computerised system is likely to pose a risk.
Join our project “Let´s Restart Together” , we can help you!
We´d like to help you in making sure your investment in time and money are fully compliant and protecting you from any potential risk.
Contact us